The Future of Organ-on-a-Chip
 5 years ago
By Bryan
5 years ago
By Bryan
Dozens of solutions have been suggested to lessen the crisis that has been hitting pharma R&D recently. Over the last decade, the cost of creating drugs has skyrocketed even as the amount of new medicines reaching market is steadily declining. Many of these potential solutions are well-known, such as the promise of AI or new models of development. But one which is less talked about and potentially more realisable is already used by many pharma professionals: organ-on-a-chip, or OOCs.
While most experts already know a great deal about where OOCs are at present, not much has been said about where they’re going. What is the future for OOC technology? How will it affect the pharma industry? Read on to find out.
What are Organs-on-Chips?
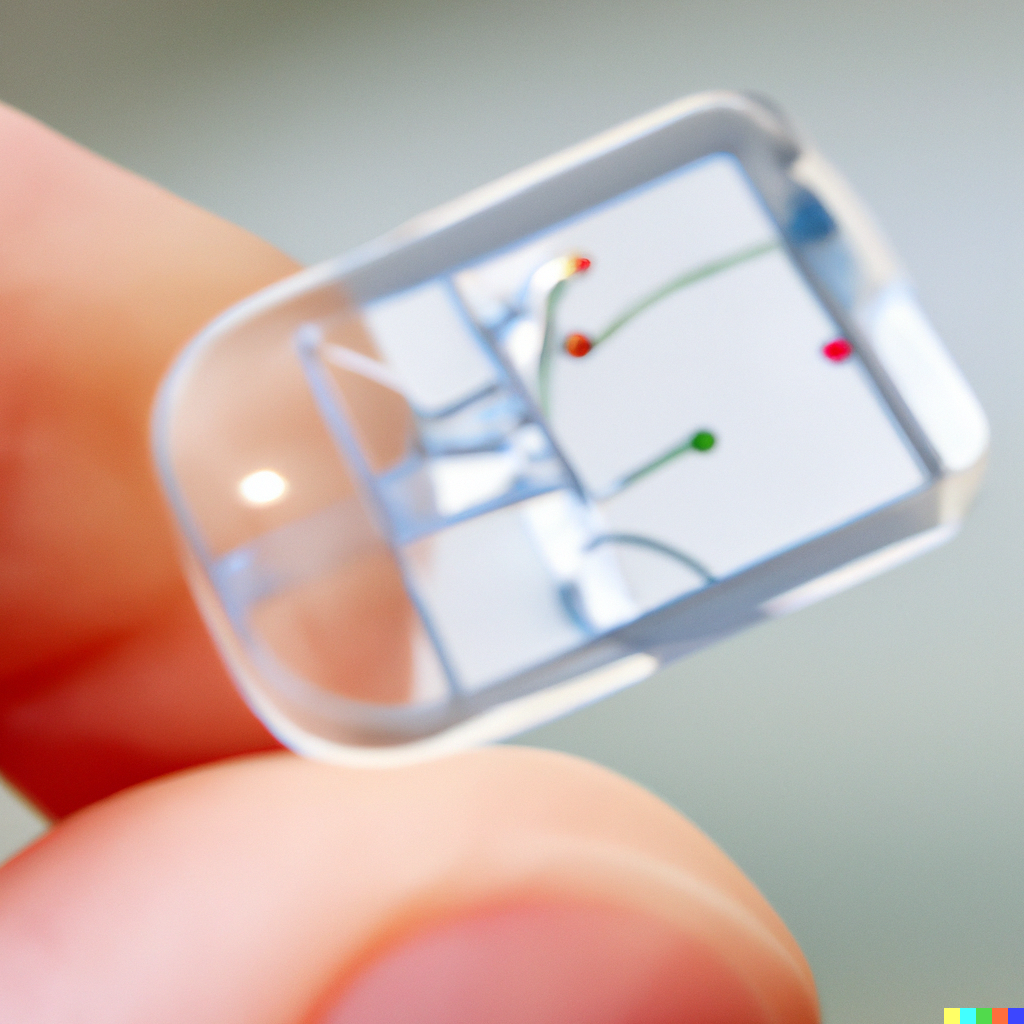
OOCs are microfluidic cell culture chips, the smallest of which are the size of a coin. These are made of a translucent polymer containing living cells able to completely simulate the microenvironment of an organ or cancer. Over the last two decades, such chips have already been made for the brain, lung, kidney, liver, gut and heart. Since 2017, however, chips have been created to combine several elements together. A 2017 article in Nature showcased an OOC comprising of a heart, liver and lung combination.
These models are created using microscale engineering technologies like replica molding and microcontact printing. A silicon-based organic polymer acts as support for tissue attachment and organisation. Their miniaturisation has a number of significant benefits for scientists, from their high analytical throughput to improved performance and facile parallelisation through multiplexing. This is without any loss in automation or precision.
There are some downsides to the several types of OOC, but most of these are far outweighed by their positives. Complex manufacturing technology is needed to create them, with integration of many functions on one chip a difficult prospect. Another particular problem with many OOCs is that there can be too few accessible cells or tissues in the system to fully study using tools such as western blotting or mass spectrometry.
Current Applications of OOCs
The applications of OOCs in pharmaceuticals are vast, primarily in the R&D space. Using OOCs to test drug candidates as early as possible saves not only time but reduces the enormous costs of prolonged trials that could end up failing. The process of developing and testing a new drug can take much more than $2 billion and an average of eleven years to complete. As such the ability to immediately test a drug in a harmless way in a biological surrogate is a huge boon for professionals, determining critical failures long before the drug would otherwise be introduced into humans (which could have potentially dangerous results).
OOCs are particularly useful where tumours are concerned. As opposed to spheroid formation models or other models used to mimic tumour microenvironments, tumour-on-a-chip models are not limited when predicting a drug’s efficacy. Instead, studying the tumour microenvironment in a controlled way and in real time allows scientists to overcome the problems of other models. It allows them to study the subject across many parameters, such as cell-to-cell and cell-to-matrix interactions within the tumour.
OOCs can be used in both preclinical R&D and clinical trials to rapidly speed up a firm understanding of a drug’s effects without risk to patients or huge investments. This is done through high-throughput screening and the mechanistic study of drugs.
The Future for OOCs
The near future for OOCs and cancer-on-a-chip models is one of streamlining and efficacy. The most recent innovations in the area involve reduction of the size of OOCs and expanding their utility towards integrating multiple body elements into one chip. Last month researchers at Kyoto University announced a new tumour-on-a-chip device the size of a coin. This can much better mimic the body’s environment than previous versions. It also helped determine that three-dimensional perfused cell culture is important for drug discovery work.
Despite the vast benefits potentially offered to the pharmaceutical industry by OOCs, the technology has only just begun to see considerable takeup in the area. Currently, the technology is still too limited to make the difference it promises. Animal models are still often the preferred tool: but coming years will see OOC models replacing current technologies, rather than simply used as an addition to aid in selection.
This will include taking on many of the factors currently incorporated into animal models, including the ability to investigate solid tumour stress and the enhanced permeability and retention (EPR) effect. One recent microfluidic model has, however, been created to incorporate tumour-like spheroids into an OOC to show the penetration of nanoparticles into tumour tissues with physiological flow conditions, validating the EPR effect in vitro.
In this vein, it has been speculated that the next generation of OOCs will potentially use cells from patients and extracted ECMs with a number of cues characterised in cancer, such as EPR effect and solid tumour stress. High detection efficiency and high-throughput technologies would also be integrated into these models to ensure better clinical relevance of OOCs to improve cancer detection.
One of the most recent developments with considerable promise is the physiologically-based perfusion in vitro system. This was created to provide as close to an in vivo cell culture environment as possible. These systems, combined with kinetic modelling, could create excellent advancements in the study of different in vitro processes.
The increasing complexity of OOCs is hoped to be building towards the first “human-on-a-chip”, a complete microfluidic system that can determine the effect of a drug on the entire human body. The closest attempt yet involved a 2D fluidic network comprising liver, lung, kidney and fat organ mimicries, with culture medium circulating as a surrogate for blood. This gave scientists an “integrated biomimetic system for culturing multiple cell types with high fidelity to in vivo situations”.
In the clinical space, OOCs will also become a wider reality in the coming years. Doctors will use the technology to culture patient cells and find suitable treatment options in vitro, before applying it to the patient.
Personalised Medicine
The drive towards personalised medicine will also see a huge uptake in the use of OOCs. Microfluidics allow the obvious advantage of being able to determine individual patients’ treatments quicker and more accurately. In addition, doctors and researchers will be able to use the technology to create “test banks” of different patient sub-populations, allowing for the repurposing of currently failing drugs and hugely reducing the difficulty and time spent in finding adequate patient populations for each trial.
That said, many believe the real efficacy of OOCs will not be achieved until their several issues are overcome. Limited present functionality and difficulty of manufacture must be removed before such a thing can be achieved. When these technicalities are surpassed, OOCs will begin to have a considerable impact across the pharmaceutical sector, from reducing the crippling time and money concerns of early R&D to speeding up clinical trials with limited risk to patients. It is safe to say that, within the next ten years, OOC technology will be a boon to almost every pharmaceutical company.

Navigating the Complex World of Global Regulatory Affairs in Oncology
In today's fast-paced global pharmaceutical landscape, the regulatory affairs sector plays a pivotal role in ensuring the safety, efficacy, and market access of oncology drugs. As the demand for innovative cancer therapies continues to grow, understanding the intricacies of global...
11 months agoNavigating the Complex World of Global Regulatory Affairs in Oncology
In today's fast-paced global pharmaceutical landscape, the regulatory affairs sector plays a pivotal role in ensuring the safety, efficacy, and market access of oncology drugs. As the demand for innovative cancer therapies continues to grow, understanding the intricacies of global...
11 months ago
Overcoming the Hurdles: Navigating the Challenges in Oncology Clinical Trials
In the world of medical research, oncology clinical trials are at the forefront of innovation and discovery. These trials play a crucial role in advancing our understanding of cancer and developing more effective treatments. However, the path to successful oncology...
11 months agoOvercoming the Hurdles: Navigating the Challenges in Oncology Clinical Trials
In the world of medical research, oncology clinical trials are at the forefront of innovation and discovery. These trials play a crucial role in advancing our understanding of cancer and developing more effective treatments. However, the path to successful oncology...
11 months ago
Embracing a Patient-Centric Approach in Oncology Trials
In the realm of healthcare and medical research, the term "patient-centric" has gained significant traction in recent years. This shift in focus towards prioritizing patients' needs and preferences is not only transforming the healthcare industry but is also making waves...
11 months agoEmbracing a Patient-Centric Approach in Oncology Trials
In the realm of healthcare and medical research, the term "patient-centric" has gained significant traction in recent years. This shift in focus towards prioritizing patients' needs and preferences is not only transforming the healthcare industry but is also making waves...
11 months ago